“In each house were three or four patients who complained of chilling, severe headaches, sweating, pain in back and extremities. After four or five relapses, the headaches and pain became unbearable for many patients who then exhibited a muddling delirium with coma, ending in death. Most were between the ages of five and 20 years. Since they are far away from even the simplest clinic, which means no possibility of saving their lives, they are dying like bees in a smoked hive.” This is an extract from a field report of the 1958 malaria epidemic in Ethiopia that killed about 150,000 people in a single season, but it could have been from the last days of the Roman Empire, whose fall has been attributed to the disease, or Ancient Egypt, or India in the 19th Century, or indeed most of the inhabited world during most of history.
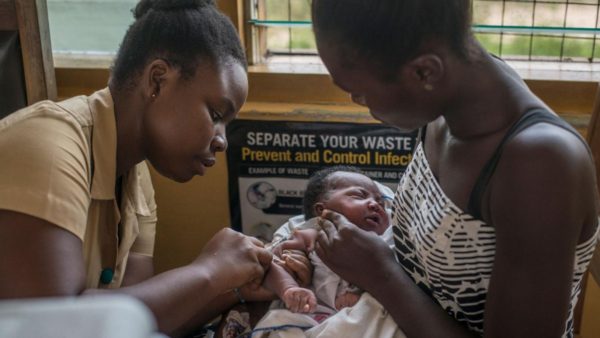
Malaria is humanity’s curse. It is among the oldest of human diseases, infecting our earliest ancestors, influencing our recent evolution, and causing an estimated half of all deaths since the Stone Age. Today, nearly half of the world’s population is at risk from malaria – it kills more than 400,000 people a year, most of them in Africa, where a child dies every two minutes from the disease. But now hopes have been raised of an end to the scourge: the first malaria vaccine is being rolled out in immunisation programmes in Malawi, Ghana and Kenya.
The new vaccine has been developed by GlaxoSmithKline with the support of the Bill & Melinda Gates Foundation, and others including the World Health Organization (WHO) and Gavi, the global vaccine alliance. It took 32 years of research, and cost more than $700m (£552m).
Trials show it to be just 40% effective at preventing the disease over four years. That’s about as effective as influenza vaccine, but considerably less than the 97% effective diphtheria vaccine. And yet, it may well be the most significant win in our war with malaria for several decades, preventing many thousands of deaths and reducing the great social and economic burden that comes with experiencing or caring for someone with chronic sickness. Public health officials in Africa are allowing themselves to become excited about the barely imagined prospect of eradicating the disease.
“I never thought there would be a vaccine in my lifetime, but now we have a chance,” says Anthony Nsiah-Asare, director general of Ghana Health Service, who is coordinating the vaccine’s implementation. “We’ve seen how it has been eradicated in some continents, so it should be possible in Africa, and now we have real hope that it could be.”

Mosquito nets are currently one of the best ways to prevent the spread of malaria (Credit: Getty Images)
Even if it is possible, we can be sure that such a foe will not be defeated easily – after all, it has spent thousands of years adapting to comfortable reliance on its human hosts.
Malaria is a disease caused by a protozoan called a plasmodium – essentially, a single-celled parasitic animal that moves around eating human tissue. Of the five different types of malaria that infect humans, Plasmodium falciparum is the most deadly. The plasmodium is spread in the bites of Anopheles mosquitoes, which transfer infected blood between people. Consequently, the disease thrives in environments where the mosquitoes thrive: warm conditions with pooled water (mosquito larvae live in water and cannot survive to adulthood without it), and plenty of human blood for the adult insects to feast on. The poorly maintained drainage in the crowded swamps around Rome were ideal, and deadly malarial epidemics caused miscarriages and killed children and adults alike, which, some historians believe, eventually brought the Empire to its knees.
Even cooler places, such as Britain, have not always been spared. Malaria, known as marsh fever, was common in the south of the country for centuries, killing thousands, and was attributed to bad air – or “mal aria” in Latin – from the marshes. The last indigenous cases were two Londoners, who contracted the disease in Stockwell as recently as 1953.
It was only in the 1890s that an army doctor living in India dissected a mosquito and managed to prove that malaria was caused by a parasite spread by the insects. European countries and others that have eliminated malaria have most often done so with environmental change – such as draining swamps, clearing slums and other mosquito breeding sites, and using insecticides and oiling standing water. Richer countries have been able to eradicate the disease through these methods, even in tropical latitudes where the mosquitoes are most efficient, such as Singapore.
However, it is in sub-Saharan Africa, where it first evolved, that the disease has proved most entrenched, historically killing half of children before the age of five. It is thought that malaria first reached epidemic proportions there with the advent of agriculture (and so, land-clearance) on the continent, around 4,000 to 5,000 years ago – there are references to malaria in Sumerian and Egyptian texts dating from 4,000 years ago. Since then, humans and protozoa have maintained an intimate relationship.
Our human bodies haven’t taken this assault unyieldingly. Over generations, affected populations have evolved various strategies to outwit the plasmodium, including by changing the shape of the blood’s oxygen-carrying haemoglobin, often at considerable cost. Sickle-cell anaemia and thalassemia, for instance, both cause weakness and reduce life-expectancy, but they also protect against potentially fatal infections of malaria, and so are far more common in exposed populations.
Multigenerational genetic strategies aside, the plasmodium has proved a tough adversary for our immune system. That’s because it is a much more complex organism than the bacteria and viruses our bodies usually fight. It is highly adaptable, so as soon as our immune system starts recognising and attacking its molecular coating (antigens), the parasite produces a different configuration to outfox us.
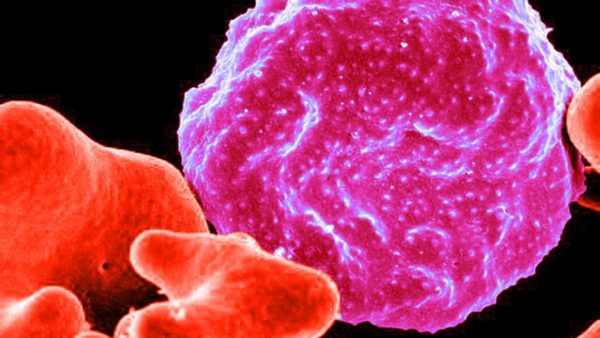
A red blood cell infected by malaria (Credit: Alamy)
The plasmodium’s most effective defence is its complicated lifecycle, during which the organism morphs into completely different forms while inside our body, each presenting different antigens to our befuddled immune system.
A bite from an infected mosquito introduces around 100 sporozoites into the victim’s blood, which circulate only for up to 30 minutes before migrating to the liver, where they hide in its cells. Here, they multiply over the next 7-10 days, morphing into merozoites, which burst out of the liver cells in protective sacs, evading the immune system. Once in the bloodstream, the sacs disintegrate and the merozoites invade the red blood cells, rapidly and exponentially multiplying and destroying the blood cells.
This destructive “blood phase” causes the periodic fevers associated with malaria. Some of the merozoites then morph into sexual forms of the parasite, male and female gametes. These will then be ingested by a biting mosquito and, within the insect’s abdomen, morph into ookinetes, which burrow into the gut wall and form oocysts. Inside the oocyst, new sporozoites form, ready to be spread by the mosquito to a new victim.
The sporozoites are only in the blood briefly and there are too few of them for the immune system to reliably recognise and mount an attack, and by the time the merozoites are circulating, the parasite is so damaging and so fast-changing that the immune system again struggles to gain control. As soon as our bodies manage a response against one type of antigen on the merozoites, the exponential explosion of new forms outdoes this effort.
So to combat each new infection, a human host must mount a new and “strain”-specific immune response to each antigenically distinct parasite in that mosquito bite, as well as the new antigens that arise during the course of the infection. Because of this, a single malarial infection can be prolonged over many months to years. Only when a sufficiently wide spectrum of parasite strains has been experienced is any immunity achieved.
Every individual exposed to endemic malaria faces a long and dangerous battle to achieve even partial immunity to malaria, and the most vulnerable are the youngest. Very young children appear to have a poor capacity to acquire effective anti-malarial immunity of any sort, but anyone who hasn’t previously experienced the endemic strain of infection also faces great threat.
Protection from invaders
Historically, this protected Africans from colonial expansion. Europeans arriving on the continent died in such great numbers that the coast of Sierra Leone was known as the White Man’s Grave. Malarial mortality rates exceeding 50% per year of contact were the norm on the West African coasts. And, despite the great cost of achieving immunity, it is readily lost – several months without reinfection are enough to leave an individual vulnerable to the full impact of malaria.
As a result, places that experience less frequent epidemics of the disease among the indigenous population can record higher death rates than those where it is endemic. Everyone I speak to in Ghana is aware of the protective benefits that come with the regular infections that they all experience. They are made more bearable through drug treatments.
The first effective treatment came from Peru. Malaria was introduced to the Americas probably in the transport of West African slaves, and indigenous people were observed treating the fevers with the bark of the Cinchona tree. In the mid-19th Century, quinine, the active antimalarial component in the bark, entered widespread use among Europeans in West Africa, rapidly reducing mortality rates by more than 75%.
In the 20th Century, a new drug chloroquine proved effective at treating and also preventing malaria, and this, in addition to the use of insecticides, such as DDT, was revolutionary. The insides and outsides of human spaces were sprayed, and malaria deaths plummeted. As prosperity increased in Europe and North America, fewer people were exposed to infection, and entire countries were declared free of the disease. In the 1950s, the dream of global eradication of malaria seemed just a decade or two away.
It was never realised. Over the decades, the malarial parasites developed resistance to chloroquine, DDT and other drugs. Society turned against insecticides because of their environmental and health impacts, banning DDT, and large human populations in the tropics have remained mired in impoverished mosquito-infested conditions. What was achieved, however, was an unprecedented reduction in the morbidity and mortality due to malaria across vast regions of the tropical and subtropical world. New drugs, such as artemisinin, which was discovered in 1972 and is used in combination with other anti-malarials to reduce parasite resistance, have made malaria a chronic rather than deadly disease for many of those with access to them.
The goal of eradication was quietly eclipsed by the aim of disease management, with an assumption that malaria will remain with us forever.

Ghana is the second country to trial the new malaria vaccine (Credit: Getty Images)
However, even when malaria doesn’t kill, repeated infections cause anaemia, weakness, body aches, dysfunction and disease of vital organs, enlargement of the spleen, infertility, miscarriage, cognitive impairment, delirium and susceptibility to other diseases. Malaria reduces length and quality of life, and in addition, it extracts a huge social and economic toll.
Caring for sick relatives and being unable to work because of fevers traps households in poverty – economists calculate that 1% negative growth each year in Africa over the last half a century can be attributed entirely to malaria. Malaria is a disease of poverty but also a disease that impoverishes.
Controlling for factors such as tropical location, colonial history, and geographical isolation, countries with intensive malaria had income levels in 1995 of only 33% that of countries without malaria, whether or not the countries were in Africa.
The burning injustice of continents of people held down by this ancient pestilence kept alive the hope for solutions. Governments and philanthropists have poured billions into the effort – last year, Bill Gates, who has made the battle against malaria a personal crusade, announced a further nearly $4bn (£3.16bn) in funding from his foundation and other donors. Much of this has gone towards highly effective mosquito-bite prevention strategies, including providing insecticide-treated bednets and eliminating breeding sites by removing or oiling standing water. But the hope of a vaccine has rumbled away in the background.
There have been many attempts at making one – all of them failing at some stage of clinical trials, falling for the same hurdles as our immune system: the tricky, shapeshifting plasmodium.
The licencing of the first proven malaria vaccine, called RTS-S, adds to our defensive arsenal and marks a significant step in the battle against the disease, enabling hopes of eradication to be raised again. The vaccine exposes the body to one of the most widely used antigens on the sporozoites, which are only in the blood briefly before secreting themselves in the liver.
Nevertheless, the hope is that by getting the immune system to attack this stage, the deadly febrile merozoites stage will be avoided in the blood. During its long development, a vaccine against hepatitis B was created, which produces a highly effective immune response, so the malarial researchers decided to add this to the sporozoite antigen in order to prime the immune system. It worked – early trials found it was 87% effective at eliminating the sporazoites.
The problem is if just one sporozoite escapes the liver and enters the blood phase as a merozoite, it has the capacity to reproduce exponentially and produce malaria. For this reason, clinical trials found that the vaccine is only 50% effective at preventing malaria for a year after inoculation, and this falls to 40% after four years.
It has been a costly, drawn out process, not without its critics, who believe the money could have been better spent on improved access to drug treatment and bednets. Malaria exerts such a terrible burden that even with this relatively low efficacy, however, that the vaccine has the potential to prevent many thousands of deaths.
The WHO has been optimistic about the project, with Matshidiso Moeti, WHO Regional Director for Africa, stating: “Innovation will enable us to outsmart the malaria parasite.” Even so, its actions are cautious, and rather than a continent-wide rollout, the project will take effect in stages, starting with pilot districts in Ghana, Malawi and Kenya this spring. The four doses needed for immunity are being added to the routine vaccination programme schedule for infants across these nations, all of which have relatively well-functioning public healthcare systems. And an accompanying education initiative urges the public to continue using their other prevention strategies, giving the programme the best chance. If it is effective, the vaccine will go global in two years’ time.
“It is a very special moment for us to see the vaccination programme begin. It’s been very emotional to see our work come to life,” says Lode Schuerman, director of global medical affairs at GSK, who has spent the past decade developing the vaccine. “It should be feasible to eliminate malaria.”
The danger, he warns, is in selecting interventions that reduce the disease incidence in some areas, but allow it to come back with deadly power against those with no immunity. “Knowing the parasite, I would use everything we have to once and for all address this burden,” he says.
This is just the start of what may be a new era of wins in the fight against malaria. Several other vaccines are also in the pipeline, including one that uses the whole sporozoites (irradiated for safety) and must be injected into a vein, but is 100% effective in laboratory trials – it will enter clinical trials next year on the island of Bioko of Equatorial Guinea.
Other researchers are targeting the mosquitoes that play such a vital role in spreading the plasmodium, looking at ways to genetically modify the insects so that they become sterile or unable to carry the parasite. Last year saw the first release of GM mosquitoes on the continent, with the introduction of 10,000 insects in Burkino Faso that carry a mutation to render the females sterile.
In Ghana, the vaccination clinics are gearing up to begin routine immunisation of malaria alongside those of other diseases, long since disappeared, such as polio and diphtheria. The warm air is thick with humidity and humming with mosquitoes. Anthony Nsiah-Asare slaps one away, and laughs when I ask if he’s ever had malaria. “No one who has been here more than a month has not been infected,” he says. “But now we will have vaccination. Now we will see the end of malaria.”
—
Gaia Vince is a writer and broadcaster specialising in science, the environment and social issues. Her first book, Adventures in the Anthropocene: A Journey to the Heart of the Planet We Made, won the 2015 Royal Society Winton Prize for Science Books. Her next book, Transcendence will be published in autumn 2019. Gaia blogs at WanderingGaia.com and tweets at @WanderingGaia.
Join one million Future fans by liking us on Facebook, or follow us on Twitter or Instagram.
If you liked this story, sign up for the weekly bbc.com features newsletter, called “The Essential List”. A handpicked selection of stories from BBC Future, Culture, Capital, Travel and Music, delivered to your inbox every Friday.